Denitrification L2
Created Mittwoch 21 Juni 2017
Basic model of a denitrification reaction.
1. Purpose of Model
Replaceable model to be used in GasVolumes:Volume_Gas_L2_Chem to simulate an ideal denitrification facility.
2. Physical Insight
This replaceable model is compatible to models of level of detail L2 according to Brunnemann et al. [1].
3. Limits of Validity
4. Interfaces
The model communicates via outer models and records. Thus its expects to have:
- an outer record named iCom as defined in Basics:Records:IComBase L2
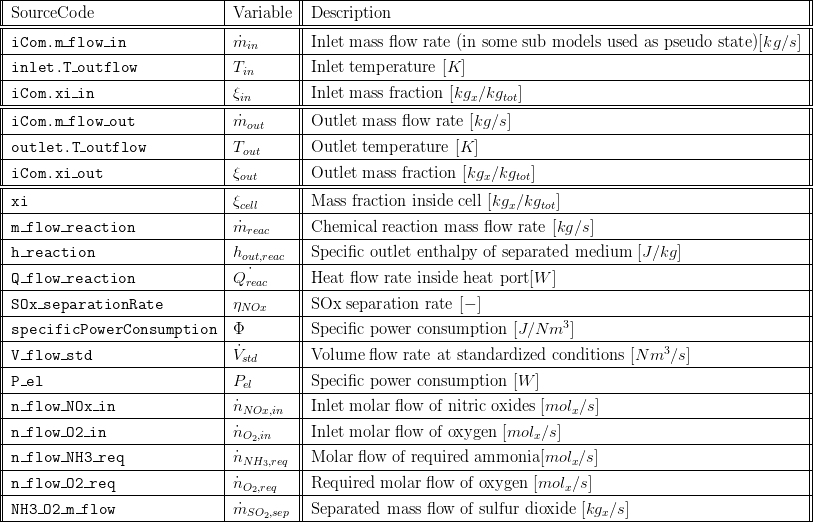
5. Nomenclature
A table referencing the nomenclature in the source code, the descriptions of variables and the "human-readable"
6. Governing Equations
Mass and energy balances are calculated in GasVolumes:Volume_Gas_L2_Chem.
The process is modelled according to the chemical equation for the conversion ofammonia, nitric oxide and oxygen to nitrogen and water:
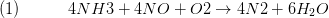
Conservation of Components
The inflowing molar flow rates are calculated with the specific component mass flow as and the molar mass. The required molar flow rates needed for the separation reaction are calculated as follows:
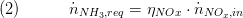
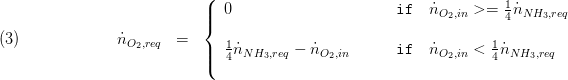
The composition is a vector of the component mass fractions. The calculation of the outlet composition depends on the type of mass conservation. If the dynamic mass balance is used, the outlet composition is calculated as follows:
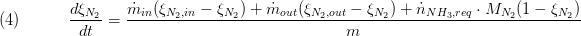
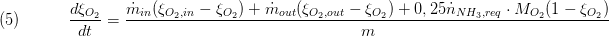

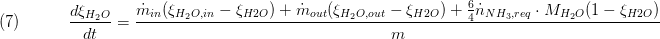
while for static mass balance the following equation is used:
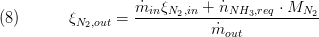
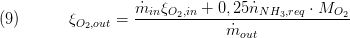
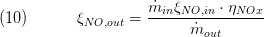
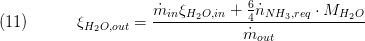
The other components are not affected by the chemical conversion an eqal the inlet composition mass flows.
The reaction mass flow equals the sum of aded ammonia and oxygen:
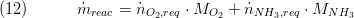
7. Remarks for Usage
8. References
[1] Johannes Brunnemann and Friedrich Gottelt, Kai Wellner, Ala Renz, André Thüring, Volker Röder, Christoph Hasenbein, Christian Schulze, Gerhard Schmitz, Jörg Eiden: "Status of ClaRaCCS: Modelling and Simulationof Coal-Fired Power Plants with CO2 capture", 9th Modelica Conference, Munich, Germany, 2012
9. Version History
- 2017 - v 1.0 - initial implementation -Lasse Nielsen, TLK Thermo GmbH
Backlinks: ClaRa:Components:FlueGasCleaning:Denitrification:Denitrification L2
